1.2 Principles of Alloy Phases, Phase Transformation, and Growth of New Phases
The magnetic properties of rare earth permanent magnetic alloys are influenced by multiple factors. Coercivity and Curie temperature can be improved by adding Co and Al. \(\text{Sm}_{2}\text{Co}_{17}\) phase segregated from the parent phase \(\text{SmCo}_{5}\) during temperature elevating from \(400^{\circ}\text{C}\) to \(750^{\circ}\text{C}\) accompanied by the precipitation of \(\text{Sm}_{2}\text{Co}_{7}\) phase.
\(\text{Sm}_{2}\text{Co}_{17}\) and \(\text{Sm}_{2}\text{Co}_{7}\) phases interact with each other during the growth process and the phase transformation is reversible when lower the temperature.
Understanding Phase in Alloys: Definition and Importance
Phase: is a phase with alike physical properties and there is an enzyme interface device to the system that fills itself within multi - phase under its ultra-high temperature and pressure is a diphase system within the is a phase system below water and steam to condense out liquid is a binary tri - phase system(Shi, 1994), and such as saturated saltwater, salt particle within it and vapor above the liquid which coexists and constitutes.
Phase Transformation: Mechanisms and Impact on Material Propertiesn
Phase transformation: it is the change in number of phases or some properties of a phase. Under the condition of certain outside restricting condition, a system making of atoms or molecules equilibrate and the system infrequently become mutually distinguished homo-genous region or recount region. Phase transformation is a macro state change, and for a phase transformation, the reduction of the free energy is the basis for determining the direction of the phase transformation. Material science and technology workers are more focused on atomic arrangement driven crystalline structure transformation, and chemical content, long range ordering, etc.; while physicists are more interested in phase transformation associated with superfluidity, superconductivity, magnetically ordered and energetically crypted ordered, etc. Phase transformation is roughly divided into first - order phase transformation and second - order phase transformation according to thermodynamics function. Phase transformation can be further categorized into diffusion phase transformation, non - diffusion phase transformation, and phase transformation of qualitative treating controlled or heat treating controlled according to atoms transfer during the formation of the new phase (Shi, 1994).).
Alloys: Composition, Structure, and Their Role in Magnetism
Alloy is a chemical combination of metals, and it is hard to demarcate “metal” and “alloy”. AlloyResearch is to use alloy as material, and it is generally to swap alloy and metal material each other..
Magnetic Materials: Fundamentals and Applications
Material is a substance to be used to manufacture useful parts of construction, apparatus and goods. According to its usage material can be divided to energy source material, construction material, and electronic material and aviation material; and as per material property characteristic it can be divided to configurable material and functional material; or can be divided to metal material and non - metallic material as per chemical component and structural features.
Material is the substance base for production and lives of human, is an important sustain of civilization of human being. Progress of material depends on progress of science and technology and social productivity, whereas progress of material also promotes progress of science and technology and development of social economy.
Alloy Phases: Formation, Stability, and Their Effect on Performance
In alloy a phase that forms uniform crystal structure and properties through interaction among component elements, has parts with homogeneous composition and is disjoined by specific interfaces is called alloy phase (Xiao, 2004). The alloy phases can be divided to four types: solid solution, pure monomeric component substance, order solid solution and metallic compound.
Solid Solution in Alloys: Structure and Magnetic Behavior
Solid material can more or less dissolve other elements without changing its structure and thus forms solid solution. Features of solid solution will change along with change of its composition. Solid solution is divided to gap solid solution and replacement solid solution as per location of solute atoms in structure of solid solution.
Exsolution Precipitation: Process and Its Impact on Magnetic Materials
The maximum dissolubility of some component in solid solution is limited, the dissolubility decreases with decreasing of temperature thus produces precipitate with solute atoms as the main component. The process to form new phase structure is called Exsolution precipitation.
Thermodynamic Basis of Phase Transformation: Principles and Classifications
Phase transformation proceeds along a path of minimum resistance and develops towards direction of energy lowing. Magnitude of free energy of the system varies
as \(\Delta G=\Delta H - T\Delta S\), if \(\Delta G < 0\), it means the system is instable thermodynamically, then diffusing and transfer occur spontaneously. Thus it can be seen that thermodynamics is the driving force for diffusion and structure transfer.
If considering one type of substance system this substance takes which structure at a certain temperature and pressure depending on its free energy to be high or low, i.e., to realize state of minimum Gibbs free energy, \(G_{min}\). Free energy \(G =\) \(H - TS\) (\(T\) is temperature; \(S\) is entropy), enthalpy \(H = E + pV\), where \(E\) is internal energy; \(p\), \(V\) are pressure and volume, in solid phase transformation of metal with little change; using the Helmholtz free energy to substitute Gibbs free energy may be more convenient, free energy of anthracite can be expressed as: \(F=\) \(E - TS\), if temperature and pressure are changed and other structure of this substance has a lower free energy then its structure will change and thus transform to be other phase, thus phase transfer or phase transformation occurs. Fig. 1.5 shows that free energy of two phases changes along with change of temperature and free energy of each phase can be written as:
\(G_{\alpha}=H_{\alpha}-TS_{\alpha}\)
\(G_{\beta}=H_{\beta}-TS_{\beta}\)
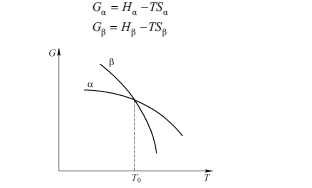
Fig 1.5 Relationship of phase transformation and system free energy
System free energy at high temperature depends on the entropy; a phase with large entropy has low free energy and is stable. The temperature at which free energy of two phases is equal is called phase transfer point or phase transition point. When temperature is higher than \(T_0\), then \(S_{\beta}<S_{\alpha}\). At \(T_0\), \(G_{\alpha}=G_{\beta}\) and \(L = H_{\alpha}-H_{\beta}=T(S_{\beta}-S_{\alpha})\). In process changing from high temperature to low temperature, the system will transform from \(\beta\) phase to \(\alpha\) phase, and discharges latent heat \(L\); in process changing from low temperature to high temperature the latent heat will transform from \(\alpha\) phase to \(\beta\) phase, and absorbs heat \(L\).
At melting point of solid material
\(L = H_{1}-H_{s}=T_{m}(S_{1}-S_{s})\)
where \(H_{1}\), \(H_{s}\), \(S_{1}\), and \(S_{s}\) represent enthalpy and entropy of liquid phase and solid phase, respectively, \(T_{m}\) represents temperature of melting point.
At phase transformation point \(T_{0}\), \(G_{\alpha}(p_{0},T_{0}) = G_{\beta}(p_{0},T_{0})\), when pressure of
system changes as \(p_{0}\to p_{0}+\Delta p\), phase transformation temperature changes as \(T_{0}\to T_{0}+\Delta T\).
Free energy \(G\) expands by Thaler progression at \((p_{0}, T_{0})\):
\(G(p_{0}+\Delta p,T_{0}+\Delta T)=G(p_{0},T_{0})+ \left(\frac{\partial G}{\partial p}\right)_{T}\mathrm{d}p + \left(\frac{\partial G}{\partial T}\right)_{p}\mathrm{d}T+\frac{1}{2}\left(\frac{\partial^{2}G}{\partial p^{2}}\right)_{T}\mathrm{d}p^{2}+\)
\(\frac{1}{2}\left(\frac{\partial^{2}G}{\partial T^{2}}\right)_{p}\mathrm{d}T^{2}+\left(\frac{\partial^{2}G}{\partial p\partial T}\right)\mathrm{d}p\mathrm{d}T+\cdots\)
(1.1)
Considering Maxwell relation formula:
\(V = \left(\frac{\partial G}{\partial p}\right)_{T}\), \(S=-\left(\frac{\partial G}{\partial T}\right)_{p}\)
Thus above formula can be expressed as:
\(G(p_{0}+\Delta p,T_{0}+\Delta T)=G(p_{0},T_{0})+V\mathrm{d}p - S\mathrm{d}T+\frac{1}{2}\left(\frac{\partial V}{\partial p}\right)_{T}\mathrm{d}p^{2}+\)
\(\frac{1}{2}\left(\frac{\partial S}{\partial T}\right)_{p}\mathrm{d}T^{2}+\left(\frac{\partial V}{\partial T}\right)_{p}\mathrm{d}p\mathrm{d}T+\cdots\)
(1.2)
Defined by homothermous compressible rate and isobaric expansible rate:
\(x =-\frac{1}{V}\left(\frac{\partial V}{\partial p}\right)_{T}\), \(\varphi=\frac{1}{V}\left(\frac{\partial V}{\partial T}\right)_{p}\)
Integral of thermal capacity ratio \(c\) by considering total sum of system entropy at various temperatures is: \(S=\int_{0}^{T}\frac{c}{T}\mathrm{d}T\), and the thermal capacity ratio at a constant pressure is \(c_{p}=T\left(\frac{\partial S}{\partial T}\right)_{p}\), thus
\(G(p_{0}+\Delta p,T_{0}+\Delta T)=G(p_{0},T_{0})+V\mathrm{d}p - S\mathrm{d}T+\)
\(\frac{1}{2}xV\mathrm{d}p^{2}-\frac{1}{2}\frac{c_{p}}{T}\mathrm{d}T^{2}+\varphi V\mathrm{d}p\mathrm{d}T+\cdots\)
(1.3)
Thus free energy of \(\alpha\) and \(\beta\) phases can be expanded in progression, and then obtains following formula by subtracting each other:
\(\mathrm{d}G=(V_{\alpha}-V_{\beta})\mathrm{d}p-(S_{\alpha}-S_{\beta})\mathrm{d}T-\frac{1}{2}(x_{\beta}V_{\beta}-x_{\alpha}V_{\alpha})\mathrm{d}p^{2}+\)
\(\frac{1}{2}\frac{c_{p\beta}-c_{p\alpha}}{T}\mathrm{d}T^{2}+(\varphi_{\beta}V_{\beta}-\varphi_{\alpha}V_{\alpha})\mathrm{d}p\mathrm{d}T+\cdots\)
(1.4)
From point of view of dynamics phase transformation is usually defined as per variation of system free energy, such as the \(n\)th - order differential coefficient
\(\frac{\mathrm{d}^{n}G}{\mathrm{d}T^{n}}\) and \(\frac{\mathrm{d}^{n}G}{\mathrm{d}p^{n}}\) of free energy vary at phase transformation point, which is defined as \(n\)th - order phase transformation (\(n - 1\) - order coefficient series).
Moore free energy function for first - order phase transformation system vs. first - order derivative function of some variables appears discontinuous phase transformation at phase transformation point. Classified guideline of this phase transformation is suggested by Ehrenfest that let Moore free energy vs. restrictive variable derivated by stages, and its derivation function appears discontinuously the lowest - order which represents the order of phase transformation. If only considering unit coefficients of two restrictive variable of temperature and intensity of pressure (\(p\)), Moore free energy (\(G\)) of each possible phase is a curve face of \(T - p - G\) space. An intersectant curve of two curve faces gives restrictive condition for equilibrium of the two phases. Moore free energy function to first - order partial derivative is \((\mathrm{d}G/\mathrm{d}T)_{p}=-S\) of temperature at a constant pressure, and Moore free energy to first - order partial derivative is \((\mathrm{d}G/\mathrm{d}p)_{T}=V\) at a constant temperature, where \(S\) and \(V\) represent Moore entropy and Moore volume, respectively. When first - order phase transformation occurs variation of Moore entropy of system is discontinuous, and \(\Delta S = \Delta H/T\), thus when first - order phase translation occurs there is latent heat effect and variation of Moore volume is discontinuous; then there is volume change along with first - order phase transformation which can be measured by means of dilatometer. First - order phase transformation usually goes along with larger heat stagnant, such as phase transformation from \(\alpha-\text{Fe}\) to \(\beta-\text{Fe}\) (Shi, 2004).
First - order phase transformation is occurred when two one - order differential items in Eq.1.4 have transformed (limited change), in Eq.1.4 one - order differential item has:
\(\left(\frac{\partial G}{\partial p}\right)_{T}=V_{\beta}-V_{\alpha}\)
\(\left(\frac{\partial G}{\partial T}\right)_{p}=S_{\beta}-S_{\alpha}\)
That is, first - order differential coefficient of free enthalpy at the point of phase transformation, in the other word, in the system there is enthalpy change (absorbing or releasing of latent heat), and also there is volume change, such as phase transformation phenomenon like melting, vaporizing, sublimating, etc.
Above formula preserves one - order differential as follows:
\(\frac{\mathrm{d}p}{\mathrm{d}T}=\frac{S_{\beta}-S_{\alpha}}{V_{\beta}-V_{\alpha}}\) (1.5)
According to definition of latent heat it can be expressed as Clapeyron equation which gives relationship between temperature and intensity of pressure of a first - order phase transformation:
\(\frac{\mathrm{d}p}{\mathrm{d}T}=\frac{L}{T(V_{\beta}-V_{\alpha})}\) (1.6)
If one of the compressible rate \(x\), heat capacity ratio \(c_{p}\), and heat expansible rate \(\varphi\) changes it will be second - order phase transformation, that is, the second - order phase transformation has no change of entropy and volume at constant temperature and constant pressure, but free enthalpy and second - order derivative of system represent discontinuous variation. Preserving quadratic item from developed formula of Thaler progression of free energy (the primary item is 0):
\(\frac{\partial^{2}G}{\partial T^{2}}=\frac{c_{p}}{2T}\) (1.7)
\(\frac{\partial^{2}G}{\partial p^{2}}=xV\) (1.8)
\(\frac{\partial^{2}G}{\partial p\partial T}=\varphi V\) (1.9)
Ehrenfest equation is derived to give relation of temperature and intensity of pressure of second - order phase transformation point:
\(\frac{\mathrm{d}p}{\mathrm{d}T}=\frac{\varphi_{\beta}-\varphi_{\alpha}}{x_{\beta}-x_{\alpha}}=\frac{c_{p\beta}-c_{p\alpha}}{T V(\varphi_{\beta}-\varphi_{\alpha})}\) (1.10)
This kind of phase transformation mainly has transformation of ferromagnetic material from ferromagnet to paramagnet at Curie temperature, transfer from conductor to superconductor at fixed temperature, transfer of some alloy material from in - order to out - of - order, etc.
Free energy function of second - order transformation system to second - order derivative function of restrictive variables is discontinuous at phase transformation point. When phase transformation occurs the corresponding physical quantity of this second - order derivative function appears discontinuous vitiated phase transformation. If discussing only the unit coefficient of two restrictive variables of temperature (\(T\)) and pressure (\(p\)) there may appears the 1st phase with its own free energy function (\(G\)) in the system, and this function is a curve face among \((G - p - T)\). The geometrical mode of intersectant two curve faces is differ from the intersectant mode of first - order phase transformation, and its free energy curve representing two phases does not intersect sharply, but is tangent along a curve. At this phase transformation point the free energy of two phases is equal and the first - order derivative of the free energy function is also equal, but the second - order derivative varies discontinuously. The second - order partial derivative of free energy function to temperature at constant pressure is \((\partial^{2}G/\partial T^{2})_{p}=\)
\(\frac{\mathrm{d}p}{\mathrm{d}T}=\frac{c_{f}^{(j)}-c_{i}^{(j)}}{TV(d_{f}-d_{i})}\) (1.14)
\(\frac{\mathrm{d}p}{\mathrm{d}T}=\frac{d_{f}-d_{i}}{\beta_{f}-\beta_{i}}\) (1.15)
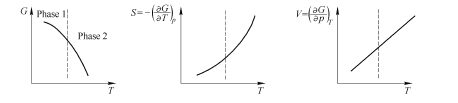
Fig. 1.6 Variation of free enthalpy, entropy, volume and heat capacity when second - order phase transformation occurs
When second - order phase transformation occurs chemical potentials and first - order partial derivatives of two phases are equal, but their second - order partial derivative is unequal. The other expressing formulae are as follows (Shi, 1994; Wang, 1980):
\(\begin{cases} \mu^{\alpha}=\mu^{\beta}\\ \left(\frac{\partial\mu^{\alpha}}{\partial T}\right)_{p}=\left(\frac{\partial\mu^{\beta}}{\partial T}\right)_{p}\\ \left(\frac{\partial\mu^{\alpha}}{\partial T}\right)_{T}=\left(\frac{\partial\mu^{\beta}}{\partial T}\right)_{T}\\ \left(\frac{\partial^{2}\mu^{\alpha}}{\partial T^{2}}\right)_{p}\neq\left(\frac{\partial^{2}\mu^{\beta}}{\partial T^{2}}\right)_{p} \end{cases}\) (1.16)
\(\begin{cases} \left(\frac{\partial^{2}\mu^{\alpha}}{\partial p^{2}}\right)_{T}\neq\left(\frac{\partial^{2}\mu^{\beta}}{\partial p^{2}}\right)_{T}\\ \left(\frac{\partial^{2}\mu^{\alpha}}{\partial T\partial p}\right)\neq\frac{\partial^{2}\mu^{\beta}}{\partial T\partial p} \end{cases}\) (1.17)
According to thermodynamics function formulae are:
\(\begin{cases} \left(\frac{\partial^{2}\mu}{\partial T^{2}}\right)_{p}=-\left(\frac{\partial S}{\partial T}\right)_{p}=-\frac{c_{p}}{T}\\ \left(\frac{\partial^{2}\mu}{\partial p^{2}}\right)_{T}=\frac{V}{V}\left(\frac{\partial V}{\partial p}\right)_{T}=-V\beta\\ \left(\frac{\partial^{2}\mu}{\partial T\partial p}\right)_{p}=\frac{V}{V}\left(\frac{\partial V}{\partial T}\right)_{p}=V\alpha \end{cases}\) (1.18)
where \(c_{p}\) is invariable heat capacity; \(A = \frac{1}{V}\left(\frac{\partial V}{\partial T}\right)_{p}\) is expanding coefficient of material; \(B=-\frac{1}{V}\left(\frac{\partial V}{\partial p}\right)_{T}\) is compressing coefficient of material.
Substitute above three formulae into Eq.1.16 and Eq.1.17 and then obtain: \(V^{\alpha}=V^{\beta},S^{\alpha}=S^{\beta}\). Classified in point of view \(c_{p}^{\alpha}\neq c_{p}^{\beta},\beta^{\alpha}\neq\beta^{\beta},\alpha^{\alpha}\neq\alpha^{\beta}\)
It can be seen that when second - order phase transformation occur heat effect and volume have no actual variation whereas volume of the phase and entropy has continuous variation. There are only expanding coefficient, heat capacity, compressing coefficient have discontinuous variation. But as \(\Delta c_{p}\neq0,\Delta\beta\neq0,\Delta\alpha\neq0\), as shown in Fig. 1.6.
The second - order phase transformations being found mainly are: transfer to superconductor state, i.e., conductor transfer to superconductor at fixed transfer temperature under non - magnetic field; some alloys transfer from in - order to out - of - order at the critical point; ferromagnet transfers to paramagnet at Curie temperature; transferring of liquid nitrogen, that is, He Ⅰ liquid transfers to He Ⅱ liquid at entering point of temperature and pressure (such as 2.19K and 5,147.31 Pa); turning of \(NH_{4}^{+}\) in crystal at fixed transfer temperature. Formula of thermodynamic different thermodynamic relation formulae can be derived, which is only applicable to uniaxial system. Whether third - order phase transformation or higher - order phase transformation found? Phase transformation of third - order or above is infrequent.
Single Crystal Alloys: Properties and Advantages
The key term for a specific type of crystal is single crystal, which is defined as a crystal that comes from a single (i.e., one) nucleus. One of its property is that it crystallography orientation can remain invariant all over its inside. And its profile may be a regular polyhedron or arbitrary irregular shape. Single crystal has inherent anisotropic properties that are fixed, and widely used in high technology field. Crystal material refers to a single crystal aspect, such as photoelectric crystal, laser crystal, acousto - optic crystal, piezoelectricity crystal, magnetic crystal film, functional crystal, etc. (Shi, 2004)
Single Crystal Superalloys: High-Performance Materials for Advanced Applications
Single crystal superalloy is developed on bases of amorphous in production of casting of superalloy and oriented crystallizing superalloy. Feature of the alloy components is not to add boundary strengthening elements, has a higher melting temperature in comparison with casting and oriented crystallizing superalloy, and has a simple form. Single crystal is manufactured by special precision casting method - selective crystallization method and seed crystal method and by using a unilateral heat flow oriented crystallization furnace. Single crystal superalloy may appear as dendrite structure, corystiform structure and plane solidification structure along with different temperature gradient and crystallization speed, this kind of alloys can effectively decrease segregation and specially have homogenous elements distribution in plane solidification structure. Use temperature of single crystal superalloy can be enhanced significantly in comparison with casting and oriented crystallized superalloy (Shi, 2004).
Enthalpy in Phase Transformations: Energy Changes and Material Stability
The enthalpy of an object system is defined as
\(H\equiv U + pV\)
where \(U\), \(p\) and \(V\) represent internal energy, pressure and volume, respectively.
According to the 1st law of thermodynamics for a system with only acting expansion work heat \(Q_{p}\) absorbed from outside in process of constant pressure equals to increment of enthalpy: \(Q_{p}=\Delta V + p\Delta V=\Delta H\). Where \(p\Delta V\) represents expansion and compressing work of system. If the system acts other works besides expansion work in process of status change, such as external magnetic field act magnetizing work to system, external force caused deformation work, etc., then a generalized enthalpy can be introduced:
\(H = V-\sum_{i}y_{i}x_{i}\)
where \(y\) is intensity, such as pressure (\(p\)), magnetic inducing intensity (\(B\)), tensile stress (\(F\)), surface tensile (\(\sigma\)), etc.; whereas \(x\) corresponds a widely extended amount, adding volume (\(V\)) total magnetic torque (\(VM, M\) as magnetization intensity), displacement (\(L\)), area (\(A\)), etc. In process of constant intensity \(Y\) system absorbs heat from external equals to increment of the generalized system enthalpy in an uniform process.
\(\mathrm{d}H=\mathrm{d}V-\sum_{i}y_{i}\mathrm{d}x_{i}=\mathrm{d}V - \mathrm{d}W=\mathrm{d}Q_{Y}\)
where \(\mathrm{d}W=\sum_{i}y_{i}\mathrm{d}x_{i}\) is the primary work in the process.
Entropy in Alloy Systems: Understanding Disorder and Stability
The entropy represents a status function of a system, and is usually represented by a common symbol \(S\). Change of entropy of a system is defined as heat absorbed of the system from external dividing temperature \(T\). External heat source in process of a particular passing, that is, \(\mathrm{d}S = \mathrm{d}Q/T\). For a reversible process there is only an infinite small amount in difference between temperature of the system and temperature of external heat source, thus \(T\) in the formula can be substituted by system temperature, whereas \(T\mathrm{d}S\) represents quantity of heat in reversible process. Unit of entropy is J/K. The system absorbs quantity of heat in a process is proportional to amount of substance containing in the system, thus entropy is an extensive parameter of additivity. It can be testified in statistical physics that entropy of system at one macro - status has relation to number \(\Omega\) of various possible micro status corresponding to that macro status, which is \(S = K\ln\Omega\). In the formula \(K = 1.381\times10^{-23}\mathrm{J}/\mathrm{K}\), which is called Boltzmann constant. The entropy represents a physical quantity of out - of - order of system state.
Latent Heat of Phase Transformation: Energy Considerations in Material Changes
Latent heat of phase transformation is that phase transformation from the matrix phase (\(\alpha\)) to a new phase (\(\beta\)) of 1 mol substance absorbs or releases heat (J/mol) within a system at constant temperature (\(T\)) and constant pressure (\(p\)). The equilibrium condition of two phases is that chemical potentials of two phases are equal.
\(\mu_{\alpha}(T,p)=\mu_{\beta}(T,p)\)
Then latent heat of phase transformation can be derived as:
\(L = T(S_{\beta}-S_{\alpha})=h_{\beta}-h_{\alpha}=\Delta h\)
where \(S\) and \(h\) represent mol entropy and mol heat enthalpy, respectively (Shi, 1994).
Driving Force of Phase Transformation: Key Factors Influencing Material Evolution
New phase (\(\beta\) phase) of unit volume generated from the matrix phase causes decrease of Gibbs free energy \(G\) at constant pressure and constant temperature. That is called the driving force of phase transformation. In condition that degree of super - cooling or superheating \(\Delta T=T_{m}-T\) is not big, then \(\Delta G_{\alpha\beta}=\) \(L[(T_{m}-T)/T]\), where \(T_{m}\) is equilibrium temperature of two phases, \(T\) is thermodynamic temperature when phase transformation takes place; \(L\) is latent heat of phase transformation of unit volume. Process of phase transformation is always in direction to decrease Gibbs free energy, the more negative the \(\Delta G_{\alpha\beta}\) is the bigger the driving force for phase transformation will be (Shi, 1994).
Growth of New Phases: Rules and Mechanisms in Alloy Development
The rules of growing up of new phase are:
- Growing up of new phase is carried out through transfer along new phase interface in solid phase transformation.
- Growth of new phase is classified to various types, there are cooperating type transfer, non - cooperating type transfer, continuous growing up and stage mechanism growing up, diffusion control and non - interface control.
- Non - diffusion phase transformation has unchanged composition; stowing sequence in transfer relies on displacement movement of interface.
- Gliding interface constituted by Shockley displacement, stowing sequence in dense alignment lattice is ABCABC on fcc lattice of dense alignment face, and on dense alignment face of hcp lattice stowing sequence is ABABAB. Actually there is stowed layer displacement in crystals and displacement exists at the edge of layer displacement, as shown in Fig. 1.7 as follows.
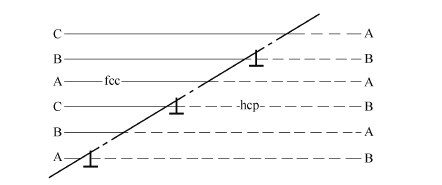
Fig. 1.7 Gliding interface constituted by Shockley displacement
It can be seen from Fig. 1.7 that there is a Shockley displacement on every other dense alignment face and a series of Shockley displacement constitutes the interface. The left side of the interface is fcc lattice and the right side is hcp lattice. The interface constituted by a series of Shockley displacement will transfer along with gliding of displacement because Shockley displacement may glide along \(y\) face (111) in direction of \(y\) (112). This type of interface is called possibly gliding interface. This result will lead to growing up of one phase and reducing of the other phase (Liu, Ren, Song, 2003).
5. Non - cooperative type transfer and growing up without change of composition.
When interface between new phase and the matrix phase is a non - lattice interface the interface of matrix phase is prone to contain the atoms from the matrix phase so that growing up can be carried out continuously, which is called continuous growing up. And because there is no composition change in growing up of new phase thus the growth only needs short distance diffusion of atoms near the interface, thus this transfer is only controlled by interface course.
Moving rate of interface becomes slowly along with decrease of temperature and will increase along with superheated degree in heat transfer (Liu, Ren, Song, 2003).
6. Non - cooperative transfer and growing up with change of composition.
Volume diffusion controlled growing up and global new phase growing up by heat activation: growing up speed of new phase is proportional to diffusion coefficient of solute in the matrix phase and concentration gradient, but the bigger the difference of composition between new and old phases is the smaller the growing up speed is. When degree of super - cooling increases concentration gradient will be raised. Diffusion coefficient takes on exponential decrease along with decrease of temperature. Decrease of temperature has two contrary function, that is, enhancing driving force and lowering diffusion ability, which leads to the maximum value on the curve of growing speed (Liu, Ren, Song, 2003).
Growing up in side of sheet new phase (Qi, 2003): growing up is not in constant speed, and growing up speed is related to diffusion coefficient of atom and the time that the bigger the diffusion coefficient is the faster the growing up speed is. But they do not appear a linear relationship. Growing up speed is slowly along with prolonging of time, the longer the time is the smaller the growing up speed is. That may be due to that the diffusion distance becomes far and far along with passing of time and interface controlling is bigger; if the interface of new and old phases is a coherency or semi - coherency interface and interface containing factor is very small and is hard to be moved the transfer can only be realized by step moving. Transfer of step needs long distance diffusion of solute atoms because different composition of the new phase and old phase. Difficulty for mechanism of step rests with source of step. One comes from heat activation which forms a two - dimension crystalline nucleus, the other comes from outcrop of screw displacement existed in new phase on the interface.






